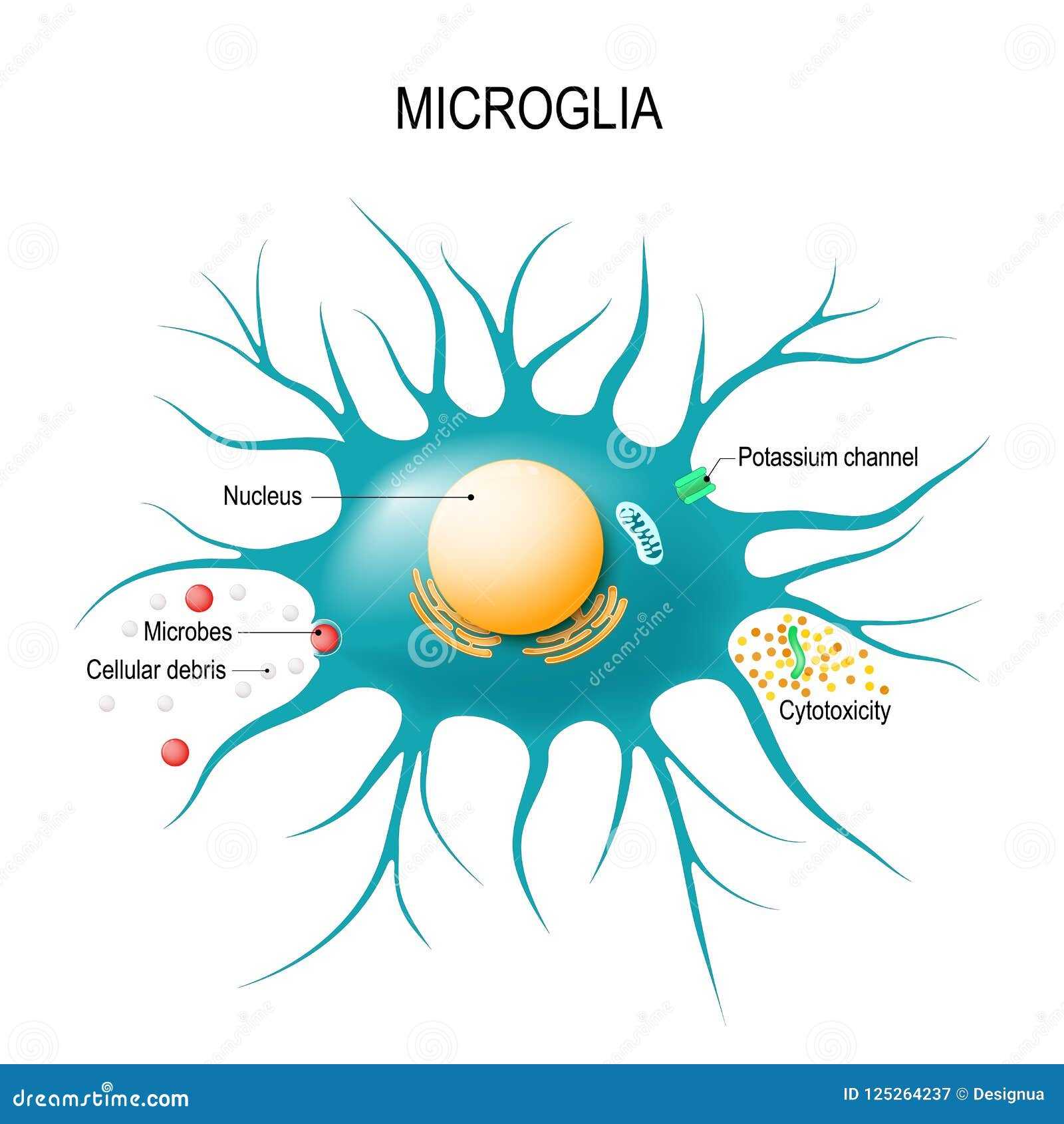
Microglial cells are a crucial component of the brain’s immune system, playing a pivotal role in maintaining neural health and homeostasis. These specialized cells continuously monitor the brain environment and respond to injury or disease by removing dead neurons and participating in synaptic pruning. This process is essential for normal brain function, but when misregulated, it can contribute to neurodegenerative diseases, including Alzheimer’s disease. Renowned neuroscientist Beth Stevens has revolutionized our understanding of these cells, revealing their dual role in both protecting and potentially damaging neural circuits. The exploration of microglial activity is leading to significant advances in the development of biomarkers and therapies for Alzheimer’s and other debilitating disorders.
In the realm of brain health, microglia serve as the first line of defense against pathogens and cellular damage. These dynamic immune cells are fundamental in ensuring the brain remains robust and functional by engaging in processes such as synaptic remodeling and the clearance of cellular debris. However, an imbalance in their activity has been implicated in conditions like Alzheimer’s disease, attesting to their influence on neurodegenerative ailments. Pioneers like Beth Stevens have shed light on the importance of the brain’s immune response, uncovering the mechanisms behind synaptic alterations and their broader implications for cognitive health. As our grasp on these brain guardians deepens, so does the potential for innovative treatments aimed at mitigating cognitive decline and enhancing neural resilience.
The Role of Microglial Cells in Brain Health
Microglial cells are integral components of the brain’s immune system, serving as vigilant guardians that maintain homeostasis by surveying the neural environment for potential threats. Their primary functions involve clearing cellular debris and pathogens, responding to infections, and participating in the process of synaptic pruning. This pruning is essential for healthy brain development, as it ensures that the synaptic connections between neurons are optimized. However, when microglia misbehave, they can inadvertently contribute to neurodegenerative diseases such as Alzheimer’s disease, leading to increased synaptic loss and cognitive decline.
Research conducted by Beth Stevens and her team at the Stevens Lab has illuminated the dual role of microglial cells in both protecting and potentially harming the brain. While their gracious role in synaptic pruning is beneficial during normal development, aberrant or excessive pruning can result in a neuroinflammatory response that exacerbates the progression of diseases like Alzheimer’s. This highlights the delicate balance microglia must maintain, underscoring the significance of their functioning in sustaining overall brain health.
Understanding Synaptic Pruning in Neurodegenerative Diseases
Synaptic pruning is a vital process that shapes the neural circuits in our brains during development and throughout life. It involves the selective elimination of synapses that are no longer needed, ensuring that only the most efficient connections remain. However, in neurodegenerative diseases such as Alzheimer’s, this process can go awry. Research from the Stevens Lab suggests that dysfunctional microglial cells may over-prune synapses, contributing to the cognitive decline seen in patients. This aberration raises critical questions about the therapeutic potential of targeting microglial activity as a means to preserve synaptic integrity.
The insights gained by studying synaptic pruning provide a pathway to developing biomarkers that can detect the early stages of Alzheimer’s disease. By understanding how microglial cells alter their behavior in response to neurodegeneration, researchers can create new treatment strategies aimed at restoring normal synaptic pruning mechanisms. This shift in focus not only transforms how we manage Alzheimer’s disease but underscores Beth Stevens’ ongoing mission to connect fundamental neuroscience research with clinical applications, ultimately improving outcomes for millions of affected individuals.
The Impact of Federal Research Funding on Alzheimer’s Studies
Beth Stevens’ path to discovering critical mechanisms of neurodegeneration was significantly enhanced by federal research funding, particularly from the National Institutes of Health (NIH). Support from governmental agencies allows researchers like Stevens to pursue their inquiries without the immediate pressure of generating commercial outcomes, enabling deeper exploration of basic scientific questions. This type of funding is essential, as it helps pave the way for groundbreaking discoveries in the brain’s immune system and its implications for diseases like Alzheimer’s.
Without the solid foundation built through NIH grants and support, it would have been challenging for Stevens and her colleagues to explore the far-reaching implications of microglial research. The research trajectory demonstrates how governmental funding can enable unexpected connections between different areas of study, ultimately leading to advancements in understanding Alzheimer’s disease. As Stevens’ work exemplifies, investment in basic science is critical for unveiling the mysteries of complex neurodegenerative conditions, ensuring that the scientific community continues to make meaningful strides in treatment and care.
Neuroinflammation’s Connection to Alzheimer’s Disease
Neuroinflammation is a central theme in the pathology of Alzheimer’s disease, characterized by the activation of microglial cells in response to neurodegenerative processes. This inflammation serves a protective role initially, but prolonged activation can lead to a hostile environment in the brain, exacerbating neuronal damage. The intricate relationship between microglia and inflammation is vital for understanding how neurodegenerative diseases develop and progress. By unraveling these connections, researchers hope to identify new therapeutic interventions that could mitigate the inflammatory responses and preserve neuronal function.
Beth Stevens’ work highlights the complexity of this interface, demonstrating that while microglial activation can be detrimental, it is also a necessary response to injury. The challenge lies in modulating this response to prevent it from contributing to the worsening of Alzheimer’s symptoms. Understanding how to achieve this balance between protective and harmful roles of microglia could lead to novel treatment strategies aimed at reducing neuroinflammation and enhancing brain health.
Innovations in Biomarkers for Alzheimer’s Disease Detection
As researchers, like those in Beth Stevens’ lab, delve deeper into the mechanisms underlying Alzheimer’s disease, the hunt for effective biomarkers gains prominence. Biomarkers are essential for early diagnosis, allowing for timely interventions that could slow disease progression. Stevens’ research focuses on the role of microglial cells in synaptic pruning, which may lead to the identification of neuroinflammatory biomarkers that indicate the presence of Alzheimer’s long before the onset of clinical symptoms.
Emerging technologies and innovative research methods are opening up avenues for the development of these biomarkers. By aggregating data from various studies on microglial activity and synaptic regulation, scientists are beginning to piece together how to accurately detect early warning signs of Alzheimer’s disease. Such progress not only represents a step forward in diagnosing and treating this debilitating condition but also exemplifies the potential of bridging fundamental research with clinical practice.
The Science of Neurodegeneration and Its Implications for Treatment
Understanding neurodegeneration, particularly in the context of Alzheimer’s disease, is crucial for developing effective treatments. The dynamics of microglial cells offer insight into how immune responses can either protect or harm neurological health. By investigating the molecular pathways involved in such conditions, researchers are equipped to design targeted therapies that could restore normal brain functions. This scientific inquiry leads to renewed hope for millions afflicted by neurodegenerative diseases.
Beth Stevens’ work significantly contributes to the framework for treatment strategies aimed at modifying microglial function to counteract neurodegeneration. By building on foundational knowledge about the brain’s immune system and its interaction with neurons, Stevens and her colleagues are poised to pioneer new therapeutic interventions that can alter disease outcomes for individuals suffering from Alzheimer’s disease and similar disorders. This integral research fosters a better understanding of the biological underpinnings of neurodegeneration, setting the stage for transformative advancements in treatment.
Elucidating the Mechanisms of Synaptic Plasticity in Learning
Synaptic plasticity, the ability of synapses to strengthen or weaken over time, is foundational to learning and memory. Microglial cells play an essential role in modulating this plasticity through their involvement in synaptic pruning. Understanding how microglia interact with neurons during different phases of learning can provide crucial insights into cognitive processes and how they’re affected in neurodegenerative diseases like Alzheimer’s. Research focusing on these dynamics could enable the development of interventions that enhance synaptic functions, potentially reversing or mitigating cognitive decline.
The intersection of synaptic plasticity and microglial activity opens exciting research avenues. Investigations into how these cells influence the formation and maturation of synapses may unveil novel mechanisms by which cognitive functions are disrupted in neurodegenerative diseases. As such research progresses, it not only promises to deepen our understanding of basic neurobiology but may also lead to practical approaches to enhance learning and memory in aging populations and those affected by neurodegeneration.
Future Perspectives in Alzheimer’s Research
As the field of Alzheimer’s research continues to evolve, the contributions of scholars like Beth Stevens signal a more nuanced understanding of the disease and its implications. Utilizing insights gained from studying microglial cells and their behavior in synaptic pruning will be vital in shaping future research directions. The ongoing investigations into neuroinflammatory processes emphasize the importance of exploring the interplay between the brain’s immune system and neural health.
The prospects for Alzheimer’s research are promising, with a burgeoning interest in targeted therapies that address the root causes of synaptic loss and dysfunction. By integrating findings from foundational studies with innovative therapeutic development, there is a potential for significant advancements in treating Alzheimer’s disease. Further exploration of microglial function and neuroinflammatory responses will undoubtedly play a key role in unveiling new strategies for prevention and management of Alzheimer’s and related neurodegenerative conditions.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells serve as the brain’s immune system and have a crucial role in Alzheimer’s disease by patrolling the brain for illness and repairing damage. They clear dead cells and assist in synaptic pruning, which can sometimes lead to aberrant pruning contributing to the progression of Alzheimer’s.
How do microglial cells affect synaptic pruning in the context of neurodegenerative diseases?
In neurodegenerative diseases like Alzheimer’s, microglial cells can improperly prune synapses. This aberrant synaptic pruning may harm communication between neurons, ultimately exacerbating the symptoms and progression of diseases such as Alzheimer’s and Huntington’s diseases.
Why are microglial cells important for understanding the brain’s immune system?
Microglial cells are essential for understanding the brain’s immune system because they are the primary immune responders in the central nervous system. Their functions include surveillance, clearance of debris, and modulating inflammation, all of which are vital in maintaining brain health and combating neurodegenerative diseases like Alzheimer’s.
What discoveries has Beth Stevens made regarding microglial cells?
Beth Stevens’ research has transformed our understanding of microglial cells, revealing their significant roles in synaptic pruning and indicating that malfunctioning microglia may play a part in Alzheimer’s disease and other neurodegenerative disorders. Her work has led to potential new biomarkers and treatment avenues.
Can microglial cells provide potential treatment targets for Alzheimer’s disease?
Yes, microglial cells offer potential treatment targets for Alzheimer’s disease. By understanding their roles in synaptic pruning and inflammation, researchers like Beth Stevens are paving the way for novel therapies aimed at correcting the dysfunctions of these immune cells, which could improve outcomes for individuals affected by Alzheimer’s.
What impact do microglial cells have on cognitive function in Alzheimer’s patients?
Microglial cells impact cognitive function in Alzheimer’s patients by their involvement in synaptic pruning and neuroinflammation. Dysregulation of microglial activity can lead to impaired synaptic communication and contribute to cognitive decline, highlighting their importance in the pathology of Alzheimer’s disease.
How has federal funding influenced research on microglial cells?
Federal funding, notably from the National Institutes of Health, has been pivotal in advancing research on microglial cells. It has enabled scientists like Beth Stevens to explore the functions of these cells and their implications in diseases like Alzheimer’s, fostering breakthroughs in understanding the brain’s immune responses.
What is the significance of aberrant microglial pruning in neurodegenerative diseases?
Aberrant microglial pruning is significant in neurodegenerative diseases because it can lead to excessive removal of synapses and disrupt neural circuits, contributing to the pathology seen in Alzheimer’s disease. Addressing this malfunction in microglial behavior presents a new avenue for therapeutic intervention.
| Key Points |
|---|
| Microglial cells act as the immune system of the brain, clearing out dead/damaged cells and pruning synapses. |
| Aberrant microglial pruning has been linked to Alzheimer’s, Huntington’s, and other neurodegenerative diseases. |
| Research from the Stevens Lab at Boston Children’s Hospital has established new biomarkers for diagnosing and treating neurodegenerative diseases. |
| Federal funding, particularly from the NIH, has been crucial for advancing research in this field. |
| Basic science and curiosity-driven research enable significant advancements in understanding and treating diseases affecting the brain. |
Summary
Microglial cells play a pivotal role in maintaining brain health as the brain’s immune defense system. Their ability to prune synapses is essential during development, but dysregulation can lead to neurodegenerative diseases like Alzheimer’s. The research conducted by Beth Stevens and her team demonstrates the importance of understanding microglial function in developing new diagnostic and therapeutic strategies. With ongoing support from federal funding, advances in this field can lead to improved care for millions affected by these disorders.